Next-Generation Vaccines and Therapies for Seasonal Influenza - Pharmacy Times
As the temperatures begin to drop and summer ends, pharmacists will gear up for yet another influenza season in the United States. The exact timing and duration of influenza season varies from year to year, with increased circulation of influenza viruses typically beginning in October and peaking between December and February.1 Compared with years past, however, influenza activity has been less predictable since the start of the COVID-19 pandemic. With the end of the national public health emergency for COVID-19 in May 2023, what can be expected as we look ahead to the 2023-2024 influenza season and beyond?2
guerrieroale - stock.adobe.com
The number of influenza cases was historically low during the 2020-2021 season. The CDC attributes the almost nonexistent influenza season to pandemic mitigation efforts, such as social distancing, masking, reduced travel, and school closures. Additionally, that season was associated with a record high number (193.8 million) of administered influenza vaccine doses in the United States.3 As many COVID-19 precautions continued into the following season, the 2021-2022 influenza season remained mild, although there were 2 waves of influenza A (H3N2) viral infections.4 Typically, when there are 2 waves of influenza activity during a season, the predominant virus during each wave is different. The 2021-2022 season was unusual, in that the predominant virus detected was the same for both waves, and influenza activity remained elevated later in the spring than during any influenza season on record.
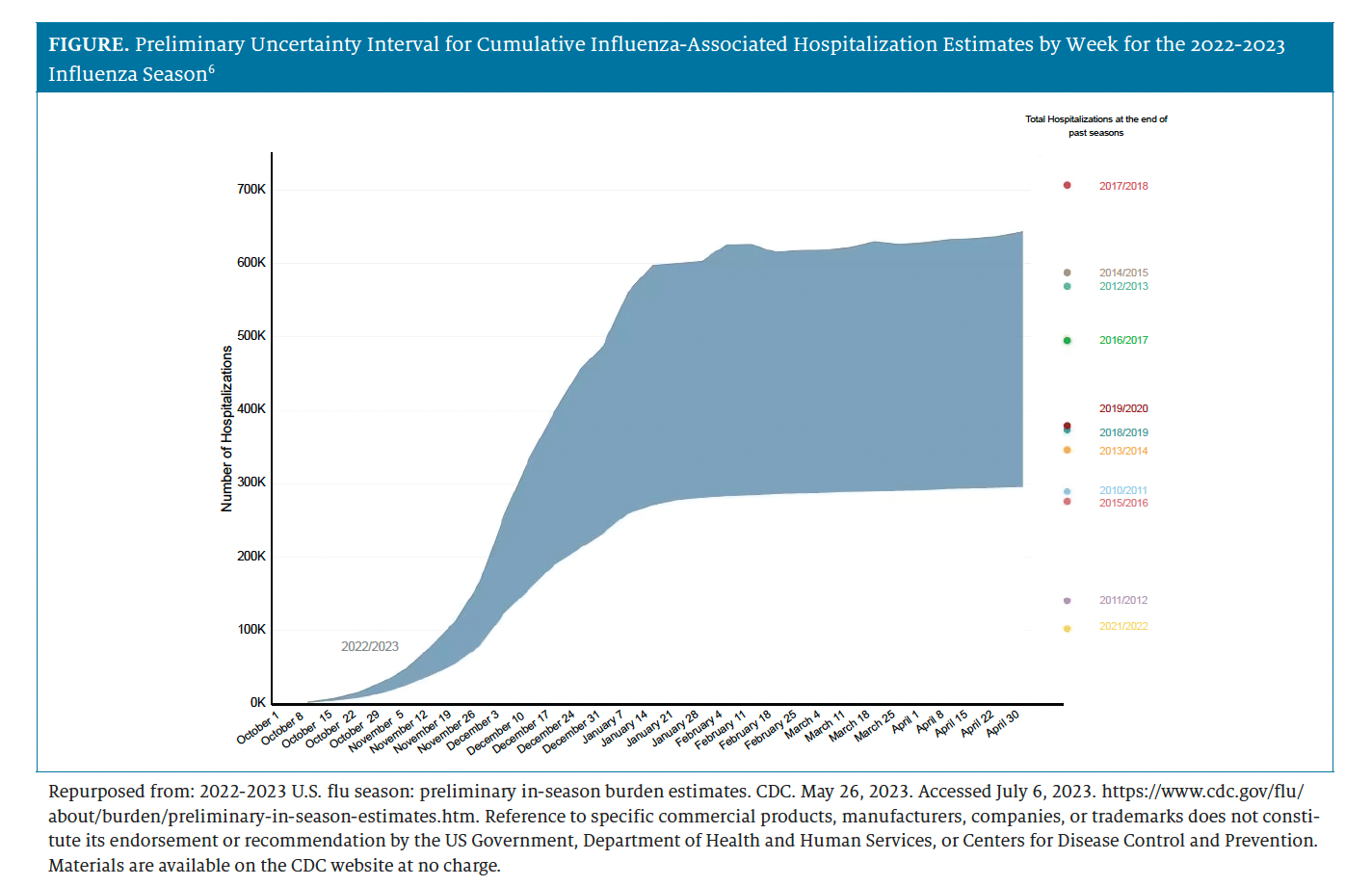
Last year, infectious disease experts cautioned US residents to prepare for the possibility of a severe 2022-2023 influenza season. Based on higher influenza burden in Australia and the Southern Hemisphere—which is as an early indicator of influenza for the Northern Hemisphere—Anthony Fauci, MD, former White House chief medical advisor, warned that residents of the United States should be prepared for a "pretty bad flu season."5 Preliminary data from the CDC for the 2022-2023 season estimated that from October 1, 2022, through April 30, 2023, there were 27 million to 54 million illnesses, 290,000 to 650,000 hospitalizations (Figure), and 19,000 to 58,000 deaths attributed to influenza infection.6 Estimates were calculated based on data collected through CDC's Influenza Hospitalization Surveillance Network; they appear as ranges, because influenza surveillance does not capture all cases of influenza that occur in the United States. Influenza A (H3N2) once again represented the majority of viral subtypes during the 2022-2023 season, but it cocirculated with influenza A (H1N1) pdm09 viruses.7 These data were preliminary, yet the final number of deaths could have been the highest recorded during the last decade.8 What remains certain is that the pre-pandemic burden of influenza has returned in force.
Figure From Pharmacy Times 2023 Influenza Guide for Pharmacists
Influenza Vaccination
Prevention remains paramount for combatting influenza and its potentially serious complications. The CDC and its Advisory Committee on Immunization Practices (ACIP) recommend routine annual influenza vaccination for all persons 6 months or older who do not present with contraindications.9 Per the CDC, the results of recent studies showed that vaccination reduced the risk of influenza illness by 40% to 60%.10 For the 2022-2023 season, vaccine protection among persons younger than 65 years was substantial, with vaccine effectiveness estimated at 54% for preventing influenza A infection that requires management by a health care provider. Similarly, the influenza vaccine was associated with 71% effectiveness in preventing symptomatic influenza A illness among children and adolescents younger than 18 years.7
Vaccine effectiveness depends on factors such as the age and health of the recipient, the type of vaccine administered, influenza virus subtype, and vaccine antigenic match with circulating viruses.11 The composition of the annual influenza vaccine is recommended by the World Health Organization (WHO), which convenes twice yearly to review influenza surveillance data and discuss candidate vaccine viruses.12 The meetings occur in February for the selection of the upcoming seasonal influenza vaccine for the Northern Hemisphere and in September for that of the Southern Hemisphere.
On February 24, 2023, the WHO announced the recommendations for viral composition of influenza vaccines for the 2023-2024 Northern Hemisphere influenza season for egg-based, cell culture–, and recombinant-based vaccines. The recommendations include strains for 2 influenza A virus subtypes (H1N1 and H3N2) and 2 influenza B virus subtypes, with an update to the A (H1N1) pdm09 strain for the 2023-2024 season.13 The FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) met the following month to review and endorse the WHO's vaccine composition recommendations for influenza vaccines in the United States.14 The factors for consideration of vaccine composition are based on global influenza virologic and epidemiologic surveillance, genetic characterization, antigenic characterization, and the candidate vaccine viruses that are available for production.14
Limitations of Current Vaccine Technology
The currently available seasonal influenza vaccine formulations are egg- and cell-based inactivated influenza vaccines, live attenuated influenza vaccines, and recombinant hemagglutinin vaccines.15 These agents provide protection against circulating virus strains that are closely related to the virus subtypes selected for the seasonal vaccine composition, but they fail to provide long-lasting and broadly protective immunity against more distantly related variants.15 Immune response following vaccination can vary between individuals, especially among those who are older or are considered at high risk of being infected (eg, pregnant women or persons with immunocompromising conditions).
Another challenge is that immunity wanes over time as levels of vaccine-specific antibodies decline, necessitating annual revaccination.16 Additionally, an estimated 2% to 10% of vaccinated, healthy individuals do not achieve adequate levels of antibodies following vaccination.15 There is an unmet need for the development of a vaccine that provides robust protection against all circulating and emerging influenza A and B subtypes and variants and that produces durable responses in high-risk populations.
Researchers have long been working toward this goal of creating a "universal influenza vaccine" that protects against all known influenza subtypes and prevents future influenza pandemics.17 With the COVID-19 pandemic driving innovation in vaccine development and deployment, many laboratory and clinical investigators are hopeful that we are closer than ever to producing an influenza vaccine that meets these criteria.
mRNA Vaccines in the Pipeline
Advances in mRNA vaccine technology took center stage during the COVID-19 pandemic, with the rapid scalability, demonstrated safety profile, robust immunogenicity, and high efficacy of nucleic acidbased mRNA vaccines emerging as a critical tool for public health.18 The mRNA platform offers several improvements over traditional vaccines, and it has the potential to deliver a new generation vaccine.19 Strain match may be more accurate without the risk of mutations associated with growing the viruses in eggs. In addition, vaccine immunity may be broader, because viral proteins are expressed at high fidelity by human cells. Furthermore, mRNA formulations allow incorporation of a larger number of antigens, which may expand protection. The next logical step is to apply this technology to the seasonal influenza challenge, and multiple pharmaceutical manufacturers currently are exploring the development of mRNA influenza vaccines.
Following their successes in bringing mRNA vaccines against SARS-CoV-2, the virus that causes COVID-19, to market, Moderna and Pfizer are the furthest along in the race to develop an mRNA influenza vaccine. Pfizer initiated a phase 3 clinical trial (NCT05540522) designed to evaluate the efficacy, safety, tolerability, and immunogenicity of a single dose of modRNA, a modified quadrivalent influenza mRNA vaccine, compared with a licensed inactivated influenza vaccine in healthy adults.20 Estimated completion of this trial is August 2023. Moderna currently is conducting research on its own investigational mRNA influenza vaccine, mRNA-1010, in a phase 3 safety and immunogenicity trial (NCT05415462) designed to compare their vaccine candidate with a licensed quadrivalent inactivated seasonal influenza vaccine in adults in the Southern Hemisphere.21,22 Moderna announced interim results from the trial on February 16, 2023, indicating that the mRNA-1010 vaccine produced superior seroconversion rates for influenza A (H3N2) and influenza A (H1N1), superiority on geometric mean titer ratios for influenza A (H3N2), and noninferiority on geometric mean titer ratios for influenza A (H1N1) when compared with the licensed quadrivalent inactivated seasonal influenza vaccine.22 Noninferiority, however, was not met for seroconversion rates and geometric mean titer ratios for the influenza B/Victoria- and B/Yamagata-lineage strains. Following these interim results, Moderna announced that they have already updated the vaccine against influenza B, and they will seek to "quickly confirm those improvements in an upcoming clinical study thanks to the agility of our mRNA platform."22 Further results of clinical trials by Pfizer, Moderna, and other companies will be watched closely to determine whether the manufacturing flexibility of mRNA vaccines (eg, greater ease to tailor vaccine to seasonal variants or variants not forecasted) can also provide greater efficacy over traditional vaccines.
Other Influenza Vaccines in the Pipeline
Novavax' vaccine candidate NanoFlu leverages nanoparticle technology with the company's proprietary Matrix-M adjuvant technology to deliver an enhanced immune response.23 In a phase 3,randomized, controlled trial (NCT04120194) in older adults (age, ≥ 65 years), NanoFlu achieved the primary end point of noninferior immunogenicity against all 4 influenza virus strains included in the vaccine candidate when compared with use of a licensed quadrivalent inactivated influenza vaccine.24 If approved, this could represent an additional vaccine option for patients in this population. The nanoparticle platform is also being explored as a universal influenza vaccine in early phase clinical trials by the National Institute of Allergy and Infectious Diseases.25
Combination Influenza/COVID-19 Vaccine
Following much public speculation about the frequency and timing of updated COVID-19 booster vaccinations, on January 26, 2023, the VRBPAC agreed with the FDA's proposal to offer a once-yearly COVID-19 booster vaccination with timing similar to that of the annual influenza vaccine—in the fall for most of the US population.26 This vaccination schedule has the potential to simplify immunization schedules for patients, but many unanswered questions remain. It is unclear whether an annual COVID-19 booster vaccination offers sufficient protection to last the full year. Unlike the seasonal nature of influenza, SARS-CoV-2 circulates year-round, and more research is needed to assess the durability of the vaccine response.27 Additionally, the concern of vaccine timing has not been addressed. Although the influenza season peaks in the late fall and winter months, the season for COVID-19 may peak at any time and thus poses the question: when is the optimal time of year to receive a COVID-19 booster vaccination?
Despite the limited evidence surrounding the timing of COVID-19 booster vaccinations, drug manufacturers are already jumping at the opportunity to combine vaccines targeting SARS-CoV-2 and influenza virus. In December 2022, Pfizer/BioNTech received Fast Track Designation from the FDA for their mRNA-based combination vaccine.28
Moderna has a combination vaccine (influenza and COVID-19) and a triple-combination vaccine (COVID-19, influenza, and respiratory syncytial virus) that are currently being assessed in phase 1/2 trials.29 They anticipate that these vaccine products, in addition to others, will launch by 2027.30
On April 20, 2023, Novavax announced positive initial results from its phase 1/2 clinical trial of their COVID-19–influenza combination vaccine candidate, which combines the NanoFlu influenza vaccine that has not yet been FDA approved with Novavax' COVID-19 vaccine.31 Although these combination vaccines are unlikely to be available for the 2023-2024 influenza season, they could revolutionize the influenza vaccination landscape in the following years.
Influenza Therapies
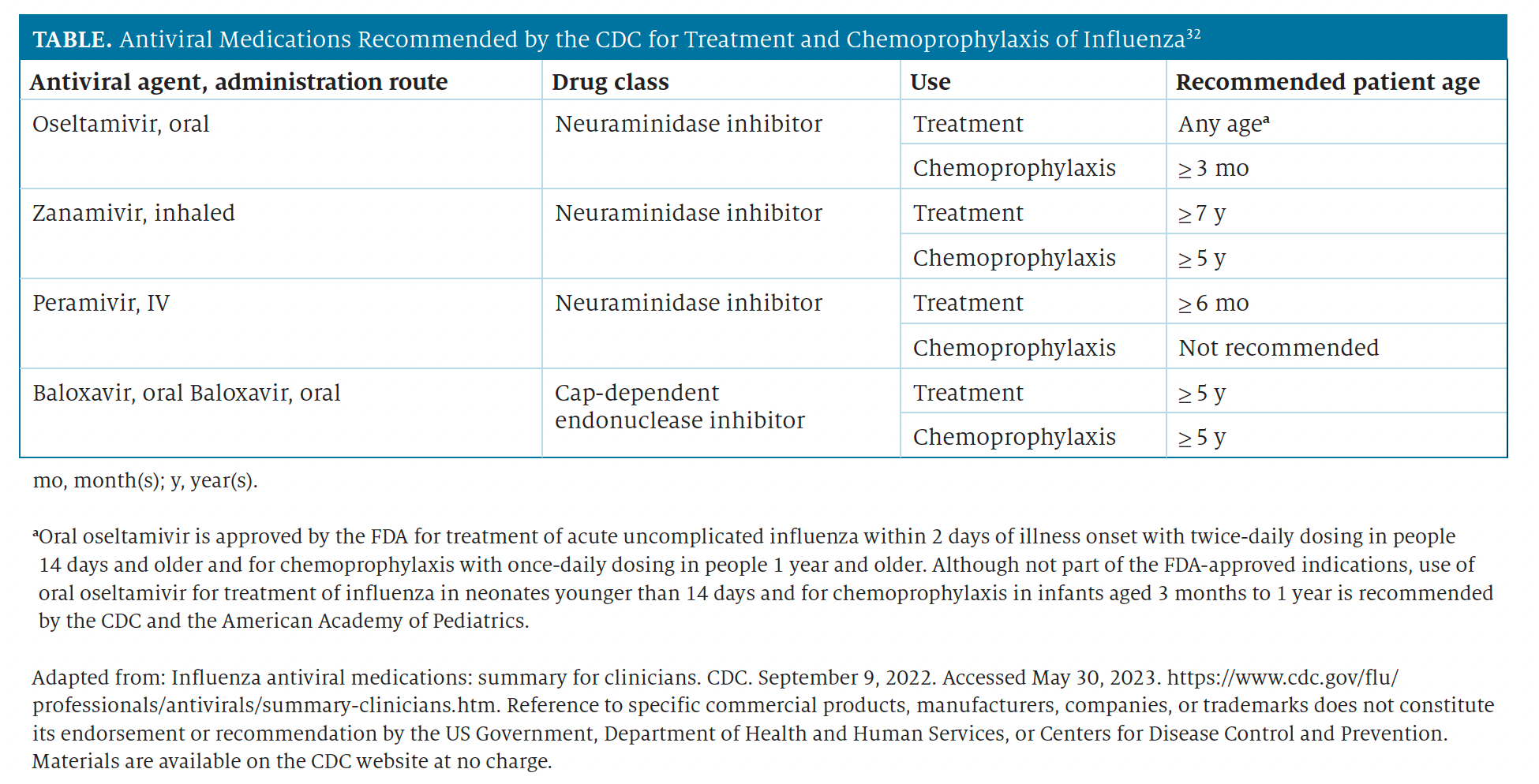
The mainstays of treatment and chemoprophylaxis for influenza infection are antiviral medications with activity against current circulating influenza viruses.32 The neuraminidase inhibitors (oral oseltamivir phosphate, inhaled zanamivir, and intravenous peramivir) and a cap-dependent endonuclease inhibitor (oral baloxavir marboxil) have activity against both influenza A and B viruses, whereas the adamantanes (amantadine and rimantadine) have high levels of resistance; since 2006, they have not been recommended for use against influenza virus.32,33 Clinical benefit is greatest when antiviral treatment or chemoprophylaxis is started as close to symptom onset or exposure as possible.32 Antiviral agents recommended by the CDC are detailed in the Table.32
Table From Pharmacy Times 2023 Influenza Guide for Pharmacists
Current surveillance data on the susceptibility of circulating influenza viruses to antivirals in the United States indicate that more than 99.9% of viruses remain susceptible to the 4 CDC-recommended antiviral agents.34 However, the emergence of antiviral drug resistance remains a constant threat, as evidenced by the detection of increased levels of resistance to oseltamivir during the 2007-2008 influenza season.35 A range of antiviral medications with differing mechanisms of action are needed to ensure that alternative treatment options exist if resistance renders the current antivirals ineffective.36
Influenza Treatments in the Pipeline
TaiGen Biotechnology is developing TG-1000, a novel pan-influenza antiviral agent that interrupts viral replication and transmission via a cap-snatching mechanism for broad-spectrum activity against influenza A, influenza B, avian influenza H7N9, and oseltamivir-resistant influenza viruses.37,38 A phase 2 trial (NCT04706468) has been completed with positive results; they demonstrated that the investigational agent can alleviate influenza symptoms and clear influenza viruses.38
Cellular Sciences/EmphyCorp is evaluating a sodium pyruvate nasal spray in the treatment of both influenza and COVID-19.39,40 Per the manufacturer, sodium pyruvate is an antioxidant that reduces inflammatory agents and improves lung function in managing asthma, chronic obstructive pulmonary disease, cystic fibrosis, and allergic rhinitis.41,42 A phase 2/3 trial (NCT04824365) is underway to study the effectiveness of the treatment in patients with COVID-19 or influenza.43
Several monoclonal antibodies (mAbs) that target the influenza virus have been developed, and they would be of value in treating drug-resistant or severe influenza.44 Over the past decade, several humanized mAbs that can bind to and neutralize a broad range of influenza A and B viruses have been evaluated in preclinical and mid-stage clinical trials.45 Currently, none of these agents have made it to late-stage clinica trial evaluation.
Conclusions
Theories on influenza prevention and treatment continue to evolve. The push for advances in vaccine technology and antiviral treatments during the COVID-19 pandemic produced a public health response that was more rapid and nimble than we had ever experienced, and we are poised to apply those principles to the influenza challenge. Influenza is not going anywhere any time soon. With patience, investment, and dedication to the cause, however, we eventually may live in a world where this infection and its serious complications no longer pose a continued threat to public health—and that day may come more quickly than any of us previously thought possible.
About the Author
Libbi Green, PharmD, is a formulary operations manager at Capital Rx in Philadelphia, Pennsylvania.
References
- Flu season. CDC. September 20, 2022. Accessed May 25, 2023. https://www.cdc.gov/flu/about/season/index.html
- HHS Secretary Xavier Becerra statement on end of the COVID-19 public health emergency. Press release. US Department of Health and Human Services. May 11, 2023. Accessed May 28, 2023. https://www.hhs.gov/about/news/2023/05/11/hhs-secretary-xavier-becerrastatement-on-end-of-the-covid-19-public-health-emergency.html
- 2020-2021 flu season summary. CDC. October 25, 2021. Accessed May 25, 2023. https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm
- 2021-2022 flu season summary. CDC. January 12, 2023. Accessed May 25, 2023. https://www.cdc.gov/flu/season/faq-flu-season-2021-2022.htm
- Fauci warns of a bad flu season brewing as he nears office exit. Press Release. Bloomberg.com. August 31, 2022. Accessed May 25, 2023. https://www.bloomberg.com/news/articles/2022-08-31/fauci-warnsof-a-bad-flu-season-brewing-as-he-nears-office-exit
- 2022-2023 U.S. flu season: preliminary in-season burden estimates. CDC. May 26, 2023. Accessed July 6, 2023. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
- McLean HQ, Petrie JG, Hanson KE, et al. Interim estimates of 2022-23 seasonal influenza vaccine effectiveness—Wisconsin, October 2022–February 2023. MMWR Morb Mortal Wkly Rep. 2023;72(8):201-205. doi:h10.15585/mmwr.mm7208a1
- Past seasons estimated influenza disease burden. CDC. October 18, 2022. Accessed May 25, 2023. https://www.cdc.gov/flu/about/burden/past-seasons.html
- Summary: 'Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2022-23.' CDC. August 23, 2022. Accessed May 25, 2023. https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm
- Vaccine effectiveness: how well do flu vaccines work? CDC. February 8, 2023. Accessed May 25, 2023. https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm
- Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices–United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71(1):1-28. doi:10.15585/mmwr.rr7101a1
- Selecting viruses for the seasonal influenza vaccine. CDC. November 3, 2022. Accessed May 25, 2023. https://www.cdc.gov/flu/prevent/vaccine-selection.htm
- Recommendations announced for influenza vaccine composition for the 2023-2024 northern hemisphere influenza season. Press release. World Health Organization. February 24, 2023. Accessed May 25, 2023. https://www.who.int/news/item/24-02-2023-recommendations-announced-for-influenza-vaccine-composition-for-the-2023-2024-northern-hemisphere-influenza-season
- FluView summary ending on March 4, 2023. CDC. Updated March 10, 2023. Accessed May 25, 2023. https://www.cdc.gov/flu/weekly/weeklyarchives2022-2023/week09.htm
- Becker T, Elbahesh H, Reperant LA, Rimmelzwaan GF, Osterhaus ADME. Influenza vaccines: successes and continuing challenges. J Infect Dis. 2021;224(12; suppl 2):S405-S419. doi:10.1093/infdis/jiab269
- Houser K, Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe. 2015;17(3):295-300. doi:10.1016/j.chom.2015.02.012
- Using mRNA technology for a universal flu vaccine. National Institutes of Health. December 6, 2022. Accessed May 25, 2023. https://www.nih.gov/news-events/nih-research-matters/using-mrna-technologyuniversal-flu-vaccine
- Neuzil KM. An mRNA Influenza vaccine - could it deliver? N Engl J Med. 2023;388(12):1139-1141. doi:10.1056/NEJMcibr2215281
- Pecetta S, Rappuoli R. mRNA, the beginning of a new influenza vaccine game. Proc Natl Acad Sci U S A. 2022;119(50):e2217533119. doi:10.1073/pnas.2217533119
- A study to evaluate a modified RNA vaccine against influenza in adults 18 years of age or older. ClinicalTrials.gov. Updated May 30, 2023. Accessed May 30, 2023. https://www.clinicaltrials.gov/ct2/show/NCT05540522
- A study of mRNA-1010 seasonal influenza vaccine in adults. ClinicalTrials.gov. Updated September 21, 2022. Accessed May 30, 2023. https://clinicaltrials.gov/ct2/show/NCT05415462
- Moderna announces interim phase-3 safety and immunogenicity results for mRNA-1010, a seasonal influenza vaccine candidate. Press release. Moderna. February 16, 2023. Accessed May 30, 2023. https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-Interim-Phase-3-Safety-and-Immunogenicity-Results-for-mRNA-1010-a-Seasonal-Influenza-Vaccine-Candidate/default.aspx
- Hu L, Lao G, Liu R, Feng J, Long F, Peng T. The race toward a universal influenza vaccine: front runners and the future directions. Antiviral Res. 2023;210:105505. doi:10.1016/j.antiviral.2022.105505
- Shinde V, Cho I, Plested JS, et al. Comparison of the safety and immunogenicity of a novel Matrix-M-adjuvanted nanoparticle influenza vaccine with a quadrivalent seasonal influenza vaccine in older adults: a phase 3 randomised controlled trial. Lancet Infect Dis. 2022;22(1):73-84. doi:10.1016/S1473-3099(21)00192-4
- NIH launches clinical trial of universal influenza vaccine candidate. National Institutes of Health. June 1, 2021. Accessed May 25, 2023. https://www.niaid.nih.gov/news-events/nih-launches-clinicaltrial-universal-influenza-vaccine-candidate
- Summary minutes. 178th Vaccines and Related Biological Products Advisory Committee. FDA. January 26, 2023. Accessed May 26, 2023. https://www.fda.gov/media/166921/download
- What's next for COVID-19 vaccines? Scientists and regulators chart a course amid uncertainty. Science. January 25, 2023. Accessed May 25, 2023. https://www.science.org/content/article/what-s-next-covid-19-vaccines-scientists-and-regulators-chart-course-amid-uncertainty
- Pfizer and BioNTech receive U.S. FDA Fast Track Designation for single-dose mRNA-based vaccine candidate against COVID-19 and influenza. Press release. Pfizer. December 9, 2022. Accessed May 25, 2023. https://www.pfizer.com/news/announcements/pfizerand-biontech-receive-us-fda-fast-track-designation-single-dose-mrnabased
- Research. mRNA pipeline. Moderna. Accessed May 28, 2023. https://www.modernatx.com/research/product-pipeline
- Moderna reports first quarter 2023 financial results and provides business updates. Moderna. May 4, 2023. Accessed May 28, 2023. https://investors.modernatx.com/news/news-details/2023/Moderna-Reports-First-Quarter-2023-Financial-Results-and-Provides-Business-Updates/default.aspx
- Initial results from Novavax' COVID-19-influenza vaccine trial are first to show feasibility of combination vaccine. Press release. Novavax. April 20, 2022. Accessed May 25, 2023. https://ir.novavax.com/2022-04-20-Initial-Results-from-Novavax-COVID-19-Influenza-Vaccine-Trial-are-First-to-Show-Feasibility-of-Combination-Vaccine
- Influenza antiviral medications: summary for clinicians. CDC. September 9, 2022. Accessed May 25, 2023. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- Antiviral drug resistance among influenza viruses. CDC. November 3, 2016. Accessed May 28, 2023. https://www.cdc.gov/flu/professionals/antivirals/antiviral-drug-resistance.htm
- Weekly U.S. influenza surveillance report. CDC. Updated April 21, 2023. Accessed May 25, 2023. https://www.cdc.gov/flu/weekly/index.htm
- Dharan NJ, Gubareva LV, Meyer JJ, et al; Oseltamivir-Resistance WorkingGroup. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301(10):1034-1041. doi:10.1001/jama.2009.294
- Holmes EC, Hurt AC, Dobbie Z, Clinch B, Oxford JS, Piedra PA. Understanding the impact of resistance to influenza antivirals. Clin Microbiol Rev. 2021;34(2):e00224-20. doi:10.1128/CMR.00224-20
- Influenza antiviral TG-1000. TaiGen Biotechnology. Accessed May 25, 2023. https://www.taigenbiotech.com/en/product/detail/TG-1000
- TaiGen enters exclusive license agreement with Joincare for TG-1000 in China. Press release. BioSpace. March 22, 2023. Accessed May 25, 2023. https://www.biospace.com/article/releases/taigen-enters-exclusive-license-agreement-with-joincare-for-tg-1000-in-china/
- Reel JM, Lupfer CR. Sodium pyruvate ameliorates influenza a virus infection in vivo. Microbiol Res. 2021;12(2):258-267. doi:10.3390/microbiolres12020018
- Sodium pyruvate nasal spray treatment of COVID-19 infection. Clinical-Trials.gov. Updated November 2, 2022. Accessed May 25, 2023. https://clinicaltrials.gov/ct2/show/NCT04824365
- Sodium pyruvate. Emphycorp. Accessed May 25, 2023. https://emphycorp.com/sodium-pyruvate-usage-with-nitric-oxide/
- N115 FDA submissions. Emphycorp. Accessed May 25, 2023. https://emphycorp.com/n115-proprietary-ingredient/
- Sodium pyruvate nasal spray treatment of COVID-19 and influenza infections. CenterWatch. Accessed May 25, 2023. https://www.centerwatch.com/clinical-trials/listings/270848/sodium-pyruvate-nasal-spray-treatment-of-covid-19-and-influenza-infections/
- Koszalka P, Subbarao K, Baz M. Preclinical and clinical developments for combination treatment of influenza. PLoS Pathog. 2022;18(5): e1010481. doi:10.1371/journal.ppat.1010481
- Sedeyn K, Saelens X. New antibody-based prevention and treatment options for influenza. Antiviral Res. 2019;170:104562. doi:10.1016/j.antiviral.2019.104562
Comments
Post a Comment